Clinical Resource
An in vitro comparative assessment of a new primary wound dressing
A. Bugedo
Advanced Medical Solutions Ltd. UK
Clinical Resource
An in vitro comparative assessment of a new primary wound dressing
A. Bugedo
Advanced Medical Solutions Ltd. UK
BACKGROUND
Dressing A – A needled non woven dressing containing fibres made of alginate and sodium carboxymethyl cellulose (NaCMC) comprising 2.3% silver nominally3.
Dressing B – A needled non woven dressing containing fibres made of NaCMC comprising 1.2% silver nominally4.
Dressing C – A needled non woven dressing containing fibres made of NaCMC comprising 1.2% silver nominally and stitchbonded with cellulosic yarns5.
METHOD
Fluid Absorption (g/cm2): (W2-W1)/A
Fluid Retention (g/cm2): (W3-W1)/A
Where:
W1: Weight of the dressing sample (g)
W2: Weight of the dressing following hydration (g)
W3: Weight of the dressing sample following the application of 40mmHg compression (g)
A: Area (cm2)
The combined wet tensile strength of the dressings was measured using a tensometer with the dressings hydrated with physiological saline solution prior to measuring the tensile force. Samples were measured in the longitudinal and transverse directions and the average was reported.
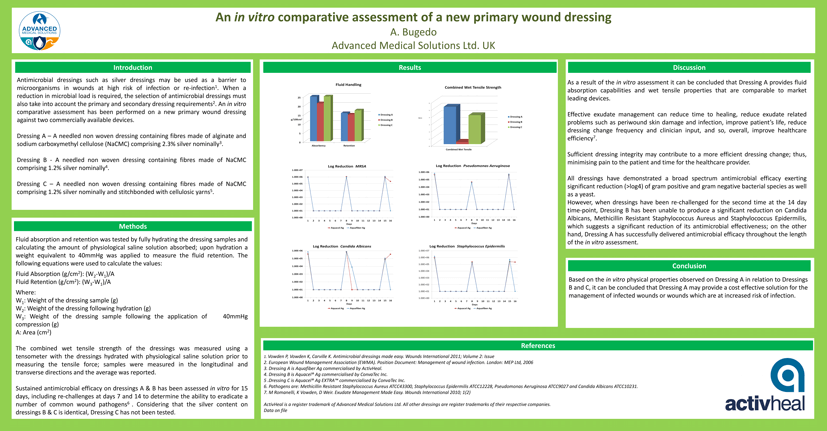
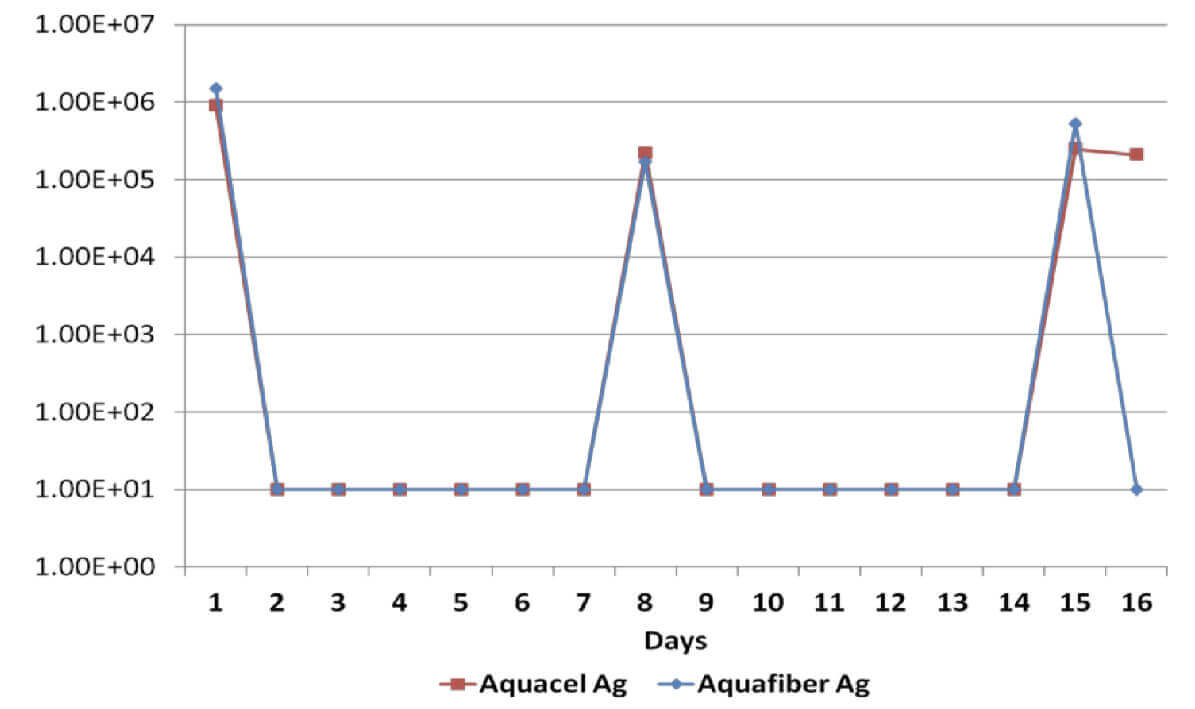
Sustained antimicrobial efficacy on dressings A & B has been assessed in vitro for 15 days, including re-challenges at days 7 and 14 to determine the ability to eradicate a number of common wound pathogens6. Considering that the silver content on dressings B & C is identical, Dressing C was not tested.
RESULTS
Fluid Handling (g/100cm2)
Combined Wet Tensile Strength (N/cm)
Log Reduction MRSA
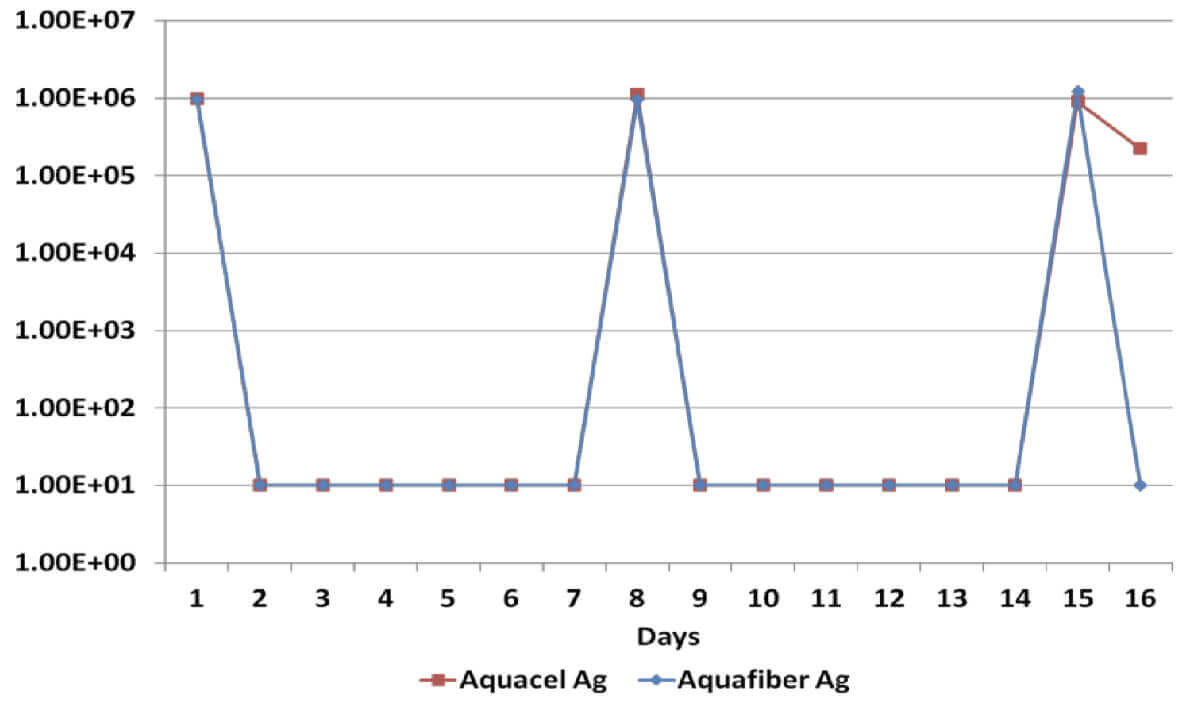
Log Reduction Pseudomonas Aeruginosa
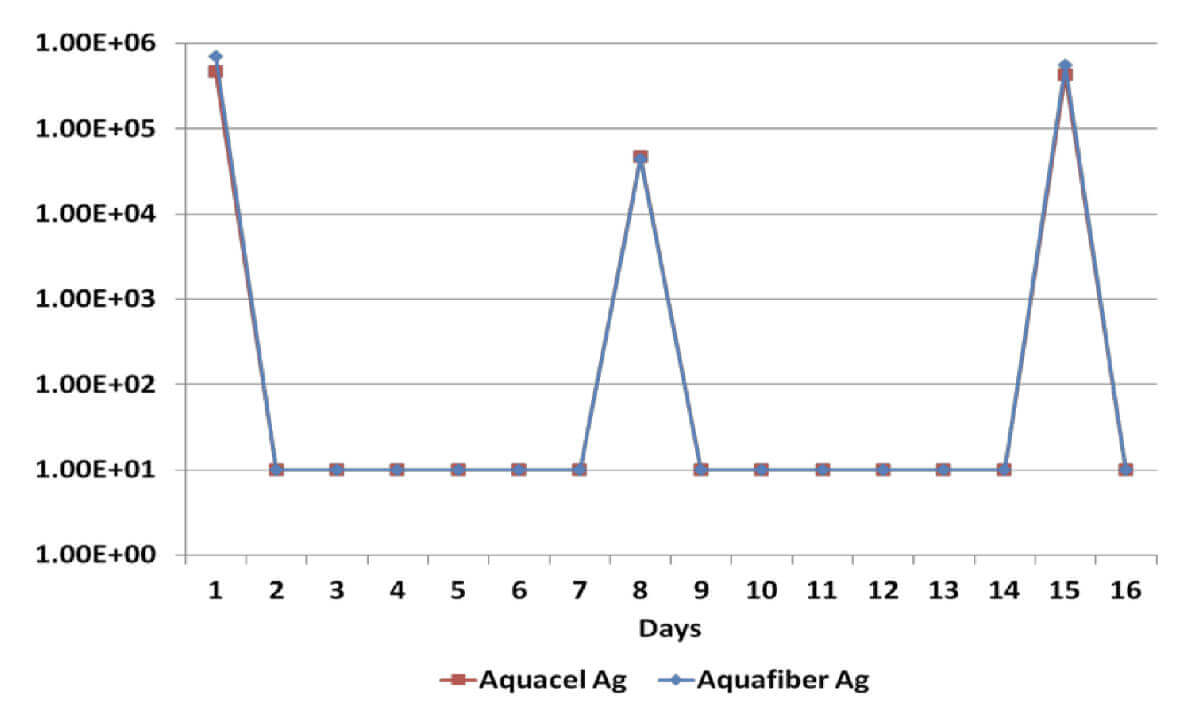
Log Reduction Candida Albicans
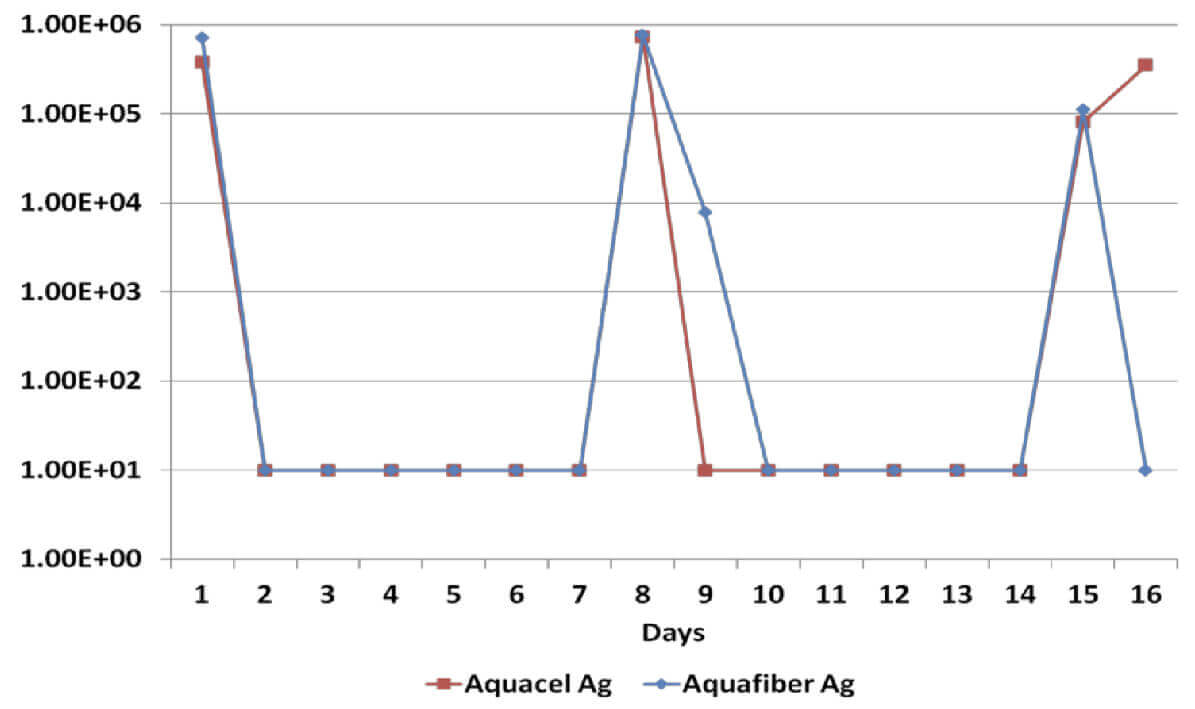
Log Reduction Staphylococcus Epidermilis
DISCUSSION
Effective exudate management can reduce time to healing, reduce exudate related problems such as periwound skin damage and infection, improve patient’s life, reduce dressing change frequency and clinician input, and so, overall, improve healthcare efficiency (7).
Sufficient dressing integrity may contribute to a more efficient dressing change; thus, minimising pain to the patient and time for the healthcare provider.
All dressings have demonstrated a broad spectrum antimicrobial efficacy exerting significant reduction (>log4) of gram positive and gram negative bacterial species as well as a yeast. However, when dressings have been re-challenged for the second time at the 14 day time-point, Dressing B has been unable to produce a significant reduction on Candida Albicans, Methicillin Resistant Staphylococcus Aureus and Staphylococcus Epidermilis, which suggests a significant reduction of its antimicrobial effectiveness; on the other hand, Dressing A has successfully delivered antimicrobial efficacy throughout the length of the in vitro assessment.
CONCLUSION
References
- Vowden P, Vowden K, Carville K. Antimicrobial dressings made easy. Wounds International 2011; Volume 2: Issue
- European Wound Management Association (EWMA). Position Document: Management of wound infection. London: MEP Ltd, 2006
- Dressing A is Aquafiber Ag commercialised by ActivHeal.
- Dressing B is Aquacel® Ag commercialised by ConvaTec Inc.
- Dressing C is Aquacel® Ag EXTRA™ commercialised by ConvaTec Inc.
- Pathogens are Methicillin Resistant Staphylococcus Aureus ATCC43300, Staphylococcus Epidermilis ATCC12228, Pseudomonas Aeruginosa ATCC9027 and Candida Albicans ATCC10231.
- M Romanelli, K Vowden, D Weir. Exudate Management Made Easy. Wounds International 2010; 1(2)
ActivHeal is a registered trademark of Advanced Medical Solutions Ltd. All other dressings are register trademarks of their respective companies. Data on file
CONTACT US FOR MORE INFORMATION
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd