ACTIVELY FIGHTING INFECTION
ACTIVHEAL® AQUAFIBER® Ag
FREE TWO WEEK EVALUATION
ACTIVELY FIGHTING INFECTION.

ACTIVHEAL AQUAFIBER® Ag
FREE TWO WEEK EVALUATION

ACTIVHEAL AQUAFIBER® Ag INDICATED FOR THE MANAGEMENT OF INFECTED WOUNDS OR WOUNDS THAT ARE AT RISK OF INFECTION.
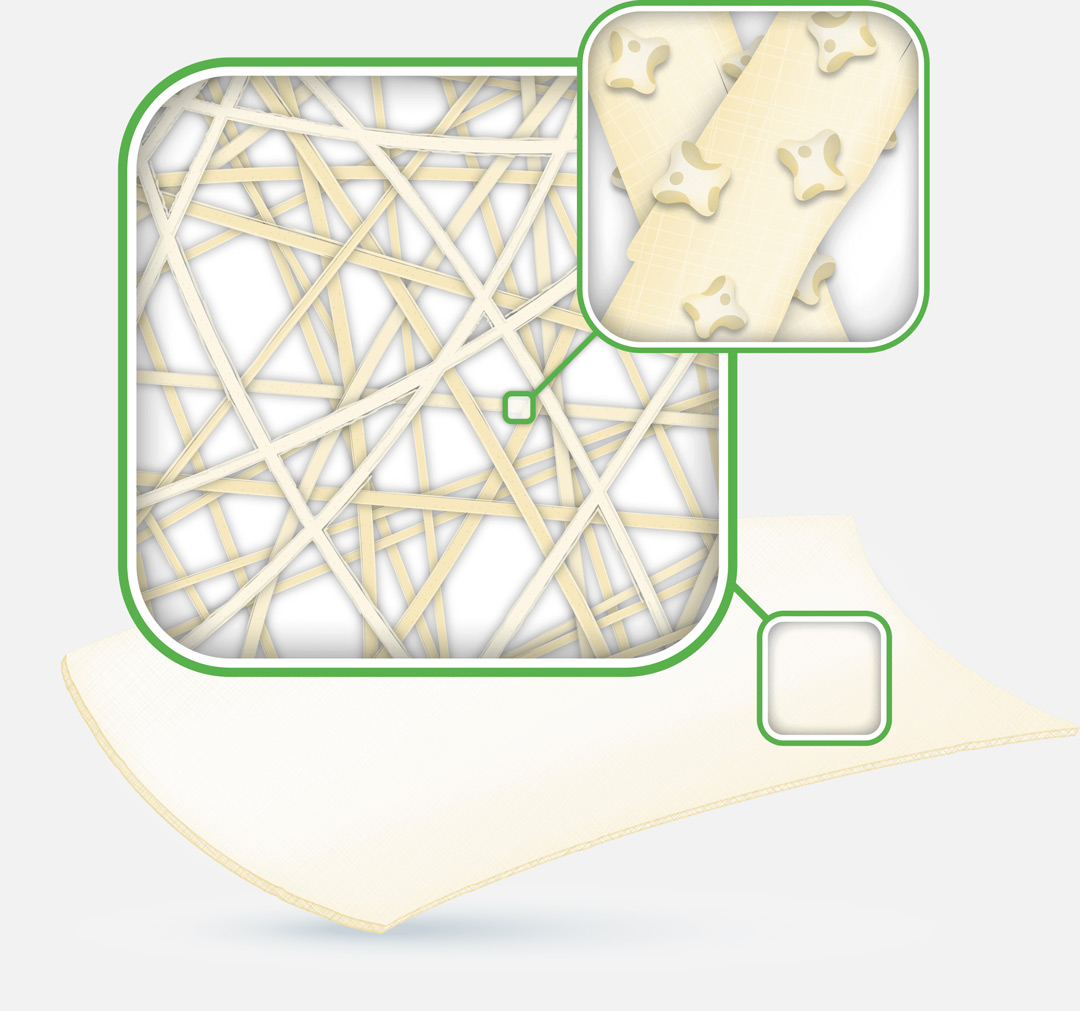
ActivHeal Aquafiber® Ag Antimicrobial wound dressing is a sterile, non-woven pad consisting of a high M (mannuronic acid) calcium alginate and carboxymethylcellulose (CMC).
Silver ions are released in the presence of wound fluid. As fluid is absorbed, the alginate forms a soft gel which assists in maintaining a moist environment for optimum wound healing and allows intact removal. Silver ions released in the presence of wound exudate are an effective antimicrobial agent for up to 7 days, based on in-vitro testing, against a broad spectrum of microorganisms frequently associated with bacterial colonisation and infection of wounds.
FEATURES
- High Silver Content
- Fast initial Elution of Silver Ions
- Sustained Silver Release
- High Wet Strength
PRODUCT RANGE
- EVERYDAY: ActivHeal Aquafiber® Ag Sheets
- SPECIALIST: ActivHeal Aquafiber® Ag Ribbon
PERFORMANCE
Absorbency1
Higher absorbency can result in reduced dressing change frequency, minimising clinician time and disruption to the patient.
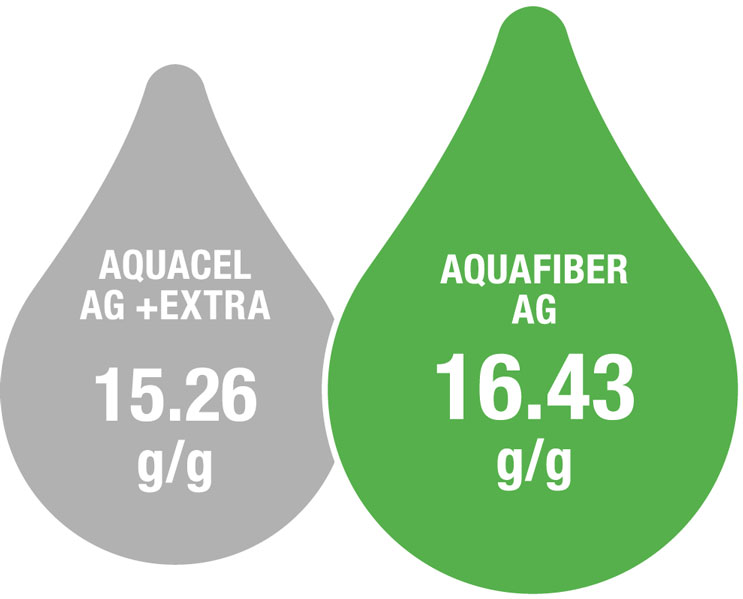
█ AquaFiber Ag
█ Aquacel Ag
█ Aquacel Ag
Fast initial and sustained anti-microbial activity 2
| AQUAFIBER AG | AQUACEL AG | |||||
|---|---|---|---|---|---|---|
| BACTERIA | 7 DAYS | 14 DAYS | 21 DAYS | 7 DAYS | 14 DAYS | 21 DAYS |
| MRSE | Yes | Yes | Yes | Yes | Yes | No |
| MRSA | Yes | Yes | Yes | Yes | Yes | No |
| VRE | Yes | Yes | Yes | Yes | Yes | No |
| S.pyogenes | Yes | Yes | Yes | Yes | Yes | No |
| S.epidermidis | Yes | Yes | Yes | Yes | Yes | No |
| E.Coli | Yes | Yes | Yes | Yes | Yes | Yes |
| C.Albicans | Yes | Yes | Yes | Yes | Yes | No |
| P.aeruginosa | Yes | Yes | Yes | Yes | Yes | Yes |
INDICATIONS
ActivHeal Aquafiber® Ag Antimicrobial wound dressing is indicated for the following moderately to heavily exuding, partial to full thickness wounds:
- Post operative wounds
- Trauma wounds (dermal lesions, trauma injuries or incisions)
- Leg ulcers
- Pressure ulcers
- Diabetic ulcers
- Partial and full thickness burns
- Cavity wounds
WOUND TYPES

Moderate > Heavy Exudate
MADE EASY VIDEOS
ACTIVELY TRY AQUAFIBER AG FOR TWO WEEKS TO SEE HOW IT FIGHTS INFECTION.
Made Easy Sessions – Brenda King from AMS – Branded on Vimeo.
Made Easy Sessions – Simon Barrett from AMS – Branded on Vimeo.
Made Easy Sessions – Valerie Edwards Jones & Pam Spruce from AMS – Branded on Vimeo.
CASE STUDY
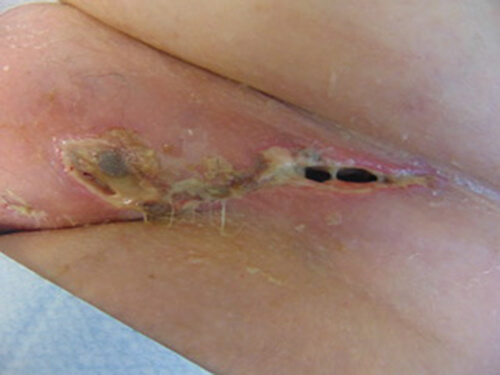
DAY 1
- Wound measured 11.2cm by 2.2cm and had a depth of 10.4cm Wound presented as 5% necrotic, 45% slough and 50% granulating tissue
- Wound showed clinical signs of infection – erythema, heat, odeama, increased level of exudate and abnormal discharge
- ActivHeal Aquafiber® Ag ribbon applied and covered with secondary dressing • Dressing was changed daily due to excessive exudates level
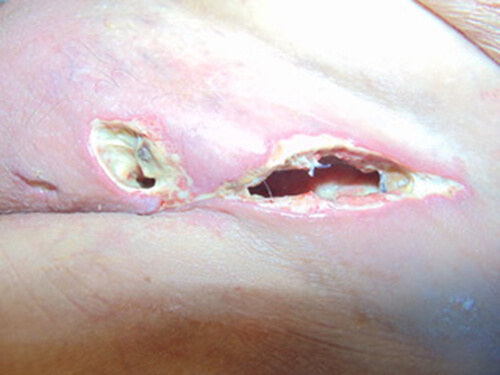
DAY 3
- All necrotic tissue removed
- Granulation tissue increased to 60%
- Infection and exudates levels reduced (bacterial burden reduction)
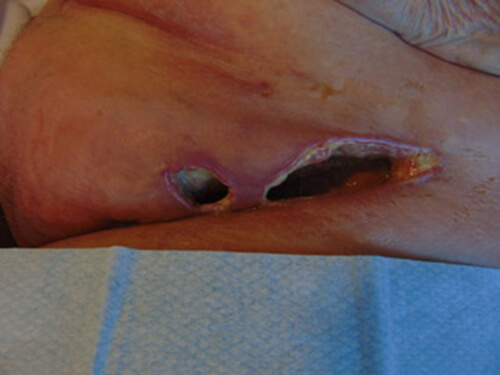
DAY 7
- 90% granulation tissue
- Wound depth reduced to 9.9cm Exudation levels reduced
- The dressing was discontinued and TNP initiated
ActivHeal Aquafiber® Ag dressing aided the reduction of bacterial bio-burden and the autolytic debridement of devitalised tissue.
ACTIVHEAL AQUAFIBER AG, PROVEN TO BE AN EFFECTIVE ANTIMICROBIAL DRESSING OF CHOICE
Clinical Evaluation: The management of exudates is an essential requirement in wound care. It is vital that an optimal moist wound environment is created and that the surrounding skin is protected from maceration and the subsequent risk of infection. ActivHeal Aquafiber Ag is a high gelling antimicrobial wound dressing that has the ability to absorb high levels of exudate whilst maintaining a moist wound environment. The addition of silver ions results in a dual purpose dressing that is proven to be effective at exudate management, as well as being effective in eradicating a broad spectrum of microorganisms including antibiotic resistant strains such as MRSA, MRSE and VRE.
FREE TWO WEEK EVALUATION
ACTIVELY TRY AQUAFIBER AG FOR TWO WEEKS TO SEE HOW IT FIGHTS INFECTION.
*Health care professionals only
* Required Field

SIZES & CODES
ActivHeal Aquafiber® Ag is available through Drug Tariff and NHS Supply Chain.
| AQUAFIBER AG | |||
|---|---|---|---|
| SIZE (CM) | QTY PER CARTON | QTY PER CASE | PRODUCT CODE |
| 5x5 | 10 | 10 | 9023236 |
| 10x10 | 10 | 10 | 9023243 |
| 15x15 | 5 | 10 | 9023267 |
| 2.7x32 Ribbon | 5 | 10 | 9023281 |
References
1. AMS Data on file: LD089-17.
2. AMS Data on file: P914R.
3. EU Market only.
ActivHeal is a Registered Trademark of Advanced Medical Solutions.
Aquacel Ag and Aquacel Ag +Extra are a registered trademark of ConvaTec.
1. AMS Data on file: LD089-17.
2. AMS Data on file: P914R.
3. EU Market only.
ActivHeal is a Registered Trademark of Advanced Medical Solutions.
Aquacel Ag and Aquacel Ag +Extra are a registered trademark of ConvaTec.
CONTACT US FOR MORE INFORMATION
* Required Field
FACTSHEET
ActivHeal® Product Range Catalogue (PDF – 19 Mb)
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd