TIME TO THINK POSITIVELY ABOUT INFECTED WOUNDS.
PHMB KILLS AND INHIBITS THE GROWTH OF BACTERIA.
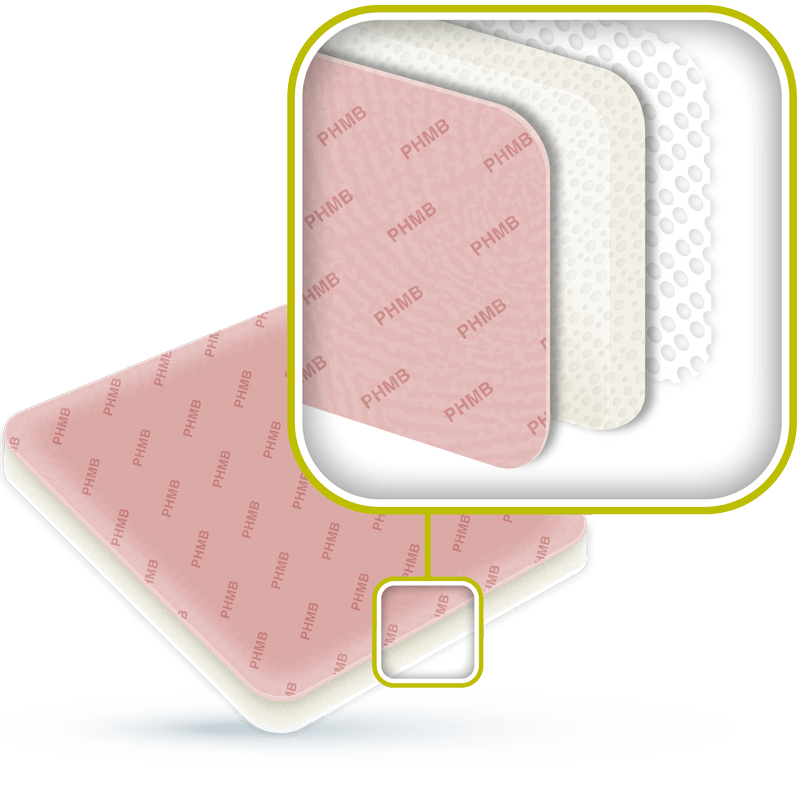
ACTIVHEAL® PHMB FOAM
FREE TWO WEEK EVALUATION
TIME TO THINK POSITIVELY ABOUT INFECTED WOUNDS.
PHMB KILLS AND INHIBITS THE GROWTH OF BACTERIA.

ACTIVHEAL® PHMB FOAM
FREE TWO WEEK EVALUATION
ACTIVHEAL® PHMB FOAM CONTAINS AN ANTIMICROBIAL WHICH KILLS & INHIBITS THE GROWTH OF BACTERIA.
FEATURES
- EXCELLENT ABSORPTION OF EXUDATE
- PROMOTES HEALING THROUGH A MOIST WOUND ENVIRONMENT
- REDUCES THE RISK OF MACERATION
- SUSTAINED ANTIMICROBIAL RELEASE AND EFFECTIVENESS
FOR UP TO 7 DAYS - BROAD SPECTRUM ANTIMICROBIAL ACTION ON MRSA, VRE, PSEUDOMONAS AERUGINOSA, CANDIDA ALBICANS
- HELPS PREVENT RECOLONISATION
- CONFORMABLE
- WATERPROOF AND BACTERIAL BARRIER
ActivHeal® PHMB Foam dressings contain the antimicrobial substance polyhexamethylene biguanide (PHMB, polyhexanide), which kills and inhibits the growth of bacteria. The PHMB is released in the presence of wound exudate and is an effective antimicrobial agent against a broad spectrum of microorganisms (gram+, gram- and yeast) that are frequently associated with the bacterial colonisation and infection of wounds.
The PHMB activity is effective for up to seven days, based on in-vitro testing.
PRODUCT RANGE
- EVERYDAY: PHMB Foam Non-Adhesive
ACTIVHEAL® PHMB HOW IT WORKS VIDEO
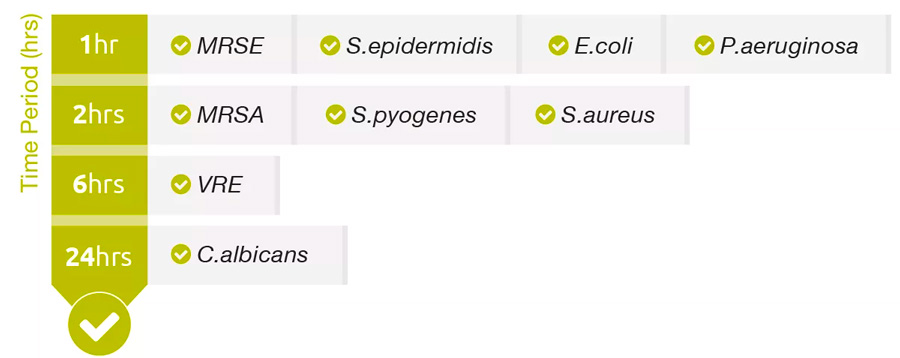
PERFORMANCE
Log Reduction2
P.aeruginosa and S.aureus.
█ Allevyn Ag
Challenge Organism3
Total Fluid Handling Performance1
handle patient exudate.
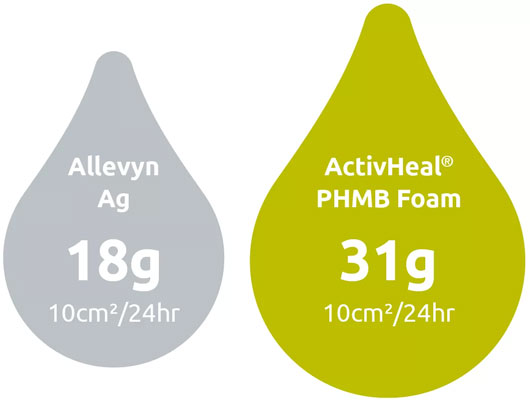
█ Allevyn Ag
INDICATIONS
- Pressure ulcers
- Leg and foot ulcers
- Diabetic ulcers
- Surgical wounds
WOUND TYPES

CONTRA-INDICATIONS
- Dry or lightly exuding wounds
- Surgical implantation
- Individuals with a known sensitivity to polyurethane films, foams, acrylic adhesive or PHMB
VIDEOS
CASE STUDY
There was 10% slough and 90% granulation to base, macerated callus to margins extending to 1st and 2nd toe cleft and moderate exudate levels. The foot showed signs of infection including heat, erythema and the patient had a raised temperature.
ActivHeal® PHMB Foam (with Systemic antibiotics) was selected to assist in reducing wound bioburden and reducing the signs and symptoms of infection in order to reduce and manage exudate levels.
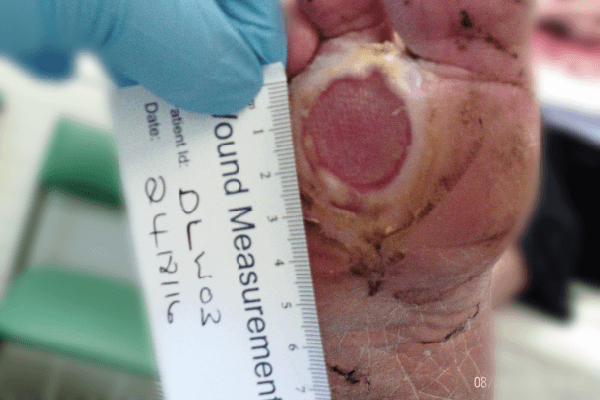
Significant progress was noted. The area of the ulcer was now 3.6cm2, a 21.8% reduction when compared to the previous assessment with no maceration. No clinical signs of infection were noted and further antibiotics were not clinically indicated. The patient reported that the dressing was comfortable, easy to apply, easy to remove and did not cause trauma to the surrounding tissue.
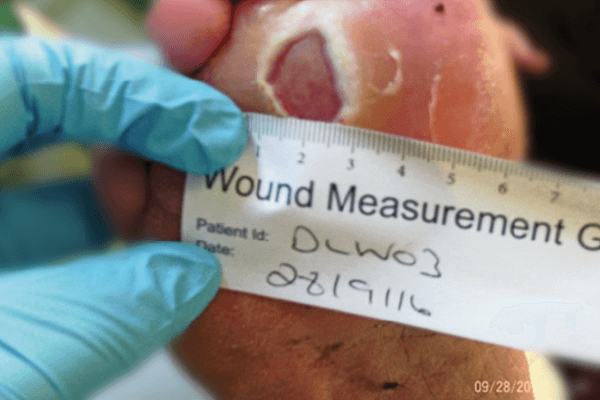
Following multiple treatments, the wound showed considerable improvement. The area of the ulcer measured 1.7cm2, a 53% reduction in size since the first application. The interdigital area had dried out and was intact. The dressing was able to provide effective exudate handling, whilst maintaining a moist wound environment and delivering antimicrobial efficacy.
ACTIVHEAL® PHMB FOAM FEATURED IN THE BRITISH JOURNAL OF NURSING
FREE TWO WEEK EVALUATION
ACTIVELY TRY PHMB FOAM FOR TWO WEEKS TO SEE HOW PHMB KILLS AND INHIBITS THE GROWTH OF BACTERIA.
*Health care professionals only
* Required Field

SIZES & CODES
| ActivHeal® | PHMB FOAM NON-ADHESIVE | |
|---|---|---|
| Size (CM) | QTY | Product Code |
| 5x5 | 10 | 9027517 |
| 7.5x7.5 | 10 | 9027524 |
| 10x10 | 10 | 9027531 |
| 10x20 | 10 | 9027548 |
| 15x15 | 10 | 9027555 |
| 20x20 | 10 | 9027562 |
AMS data on file – P2633R & P2999R.
1. AMS data on file LD017 &P2412.
2. AMS Data on File P2999R.
3. AMS Data on File P2999R. Allevyn Ag is a registered trademark of Smith and Nephew.
DOWNLOADS
CONTACT US FOR MORE INFORMATION
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd