THIS FOAM IS AN
EFFECTIVE BACTERIAL BARRIER.
ACTIVHEAL® FOAM ADHESIVE
FREE TWO WEEK EVALUATION
THIS FOAM IS AN EFFECTIVE BACTERIAL BARRIER.

ACTIVHEAL® FOAM ADHESIVE
FREE TWO WEEK EVALUATION
ACTIVHEAL® FOAM ADHESIVE IS AN ABSORBENT FOAM DRESSING IDEAL FOR MODERATE TO HIGH EXUDING WOUNDS.
FEATURES EXCELLENT ABSORPTION OF EXUDATE // VERSATILE // PROMOTES HEALING THROUGH A MOIST WOUND ENVIRONMENT // REDUCES THE RISK OF MACERATION // WATERPROOF // BACTERIAL BARRIER // SOFT AND COMFORTABLE //
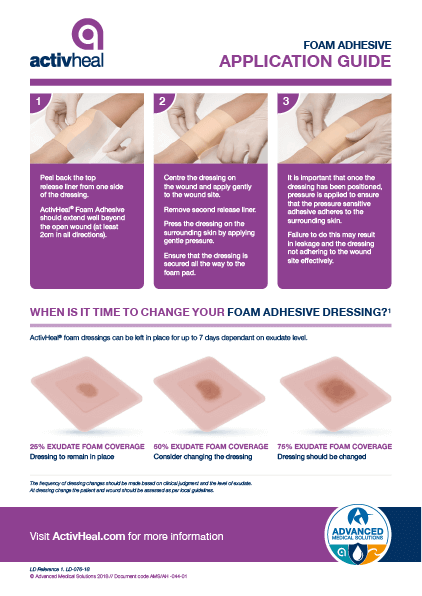
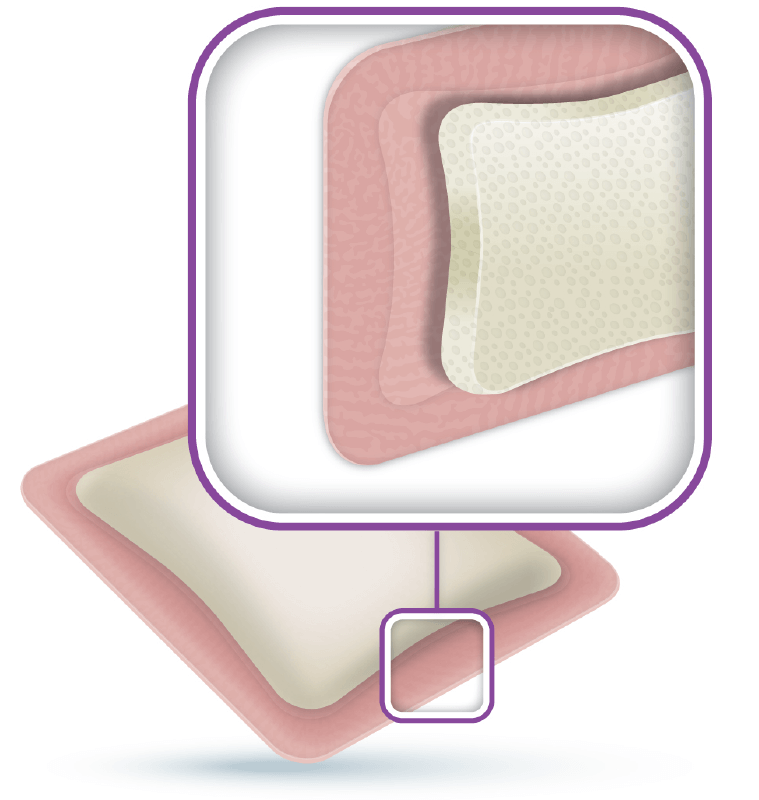
ActivHeal® Foam Adhesive is a two layer dressing indicated for moderate to heavily exuding wounds. Each layer of the ActivHeal® Foam Adhesive contributes to the performance of the dressing.
The dressing comprises of a polyurethane absorbent foam pad and a polyurethane membrane. The core of the dressing is a layer of absorbent polyurethane foam which absorbs wound exudate vertically into the dressing. The absorbent pad retains the exudate within the dressing preventing the exudate from re-entering the wound and preventing maceration to the peri wound and surrounding skin. The polyurethane membrane provides an effective barrier function and is waterproof whilst allowing the transpiration of exudate which aids the total fluid handling capacity of the dressing.
Indicated for moderate to heavily exudating wounds. The dressing offers a pressure sensitive acrylic adhesive border ensuring the dressing remains in place allowing the patient to continue everyday activities confidently. The dressing conforms to the contours of the body which reduces the risk of rucking or catching on clothing and bedding.
PERFORMANCE

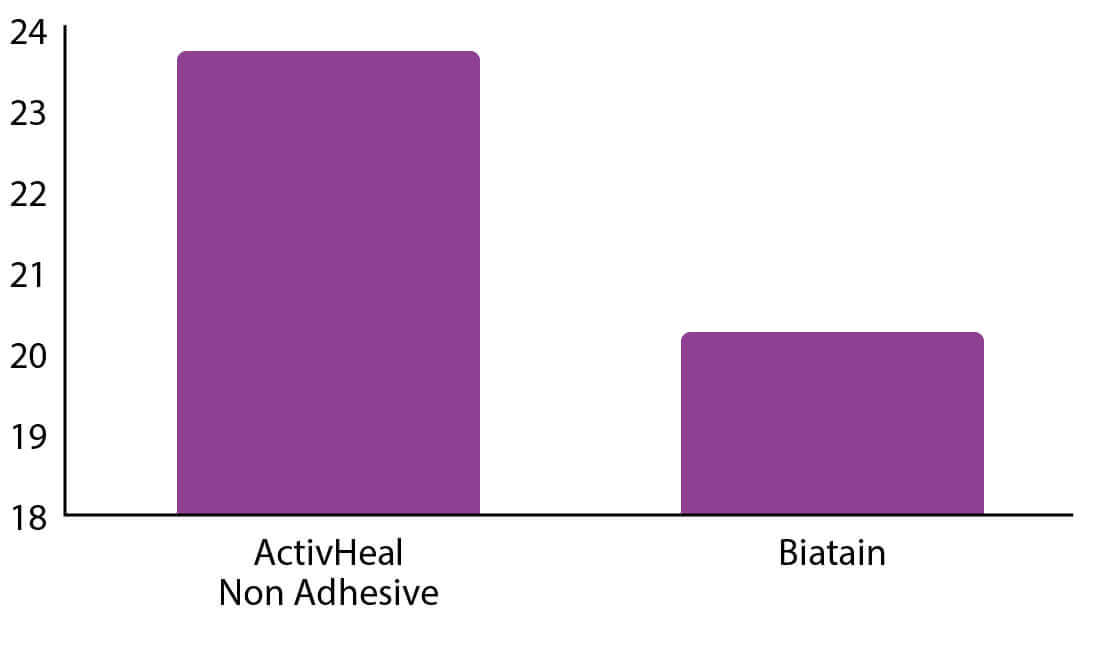
█ Absorbency
PRODUCT RANGE
- EVERYDAY: Adhesive Foam Border
INDICATIONS
- Pressure ulcers
- Leg ulcers
- Diabetic ulcers
- Post operative surgical
- Cavity wounds (as a secondary dressing)
- Lacerations and abrasions
- Graft wounds and Donor sites
- Superficial and partial thickness burns
WOUND TYPES

CASE STUDY
SIZES & CODES
| ActivHeal® | FOAM ADHESIVE | |
|---|---|---|
| Size (CM) | QTY | Product Code |
| 7.5x7.5 | 10 | 10010232 |
| 10x10 | 10 | 10009109 |
| 12.5x12.5 | 10 | 10009110 |
| 15x15 | 10 | 10009111 |
| 20x20 | 10 | 10009112 |
1. SMTL Independent Data Report No. 10/3421/1.
Permafoam is a registered trademark of Paul Hartmann Limited.
CONTACT US FOR MORE INFORMATION
DOWNLOADS
THIS FOAM IS AN EFFECTIVE BACTERIAL BARRIER.

ACTIVHEAL® FOAM NON-ADHESIVE
FREE TWO WEEK EVALUATION
THIS FOAM IS AN
EFFECTIVE BACTERIAL BARRIER.
ACTIVHEAL® FOAM NON-ADHESIVE
FREE TWO WEEK EVALUATION
ACTIVHEAL® FOAM NON-ADHESIVE CREATES A MOIST WOUND HEALING ENVIRONMENT.
FEATURES EXCELLENT ABSORPTION OF EXUDATE // VERSATILE // PROMOTES HEALING THROUGH A MOIST WOUND ENVIRONMENT // LOW FRICTION BACKING // LOW ADHERENT WOUND CONTACT LAYER // SOFT AND COMFORTABLE // IDEAL UNDER COMPRESSION //
PERFORMANCE
PRODUCT RANGE
- EVERYDAY: Non-Adhesive Foam Border & Non-Adhesive Foam Non Border
- SPECIALIST: Non-Adhesive Foam Heel, Tracheostomy
INDICATIONS
- Pressure ulcers
- Leg ulcers
- Diabetic ulcers
- Post operative surgical
- Superficial and partial thickness burns
- Lacerations and abrasions
- Graft wounds and Donor sites
- Cavity wounds (as a secondary dressing)
WOUND TYPES

CASE STUDY
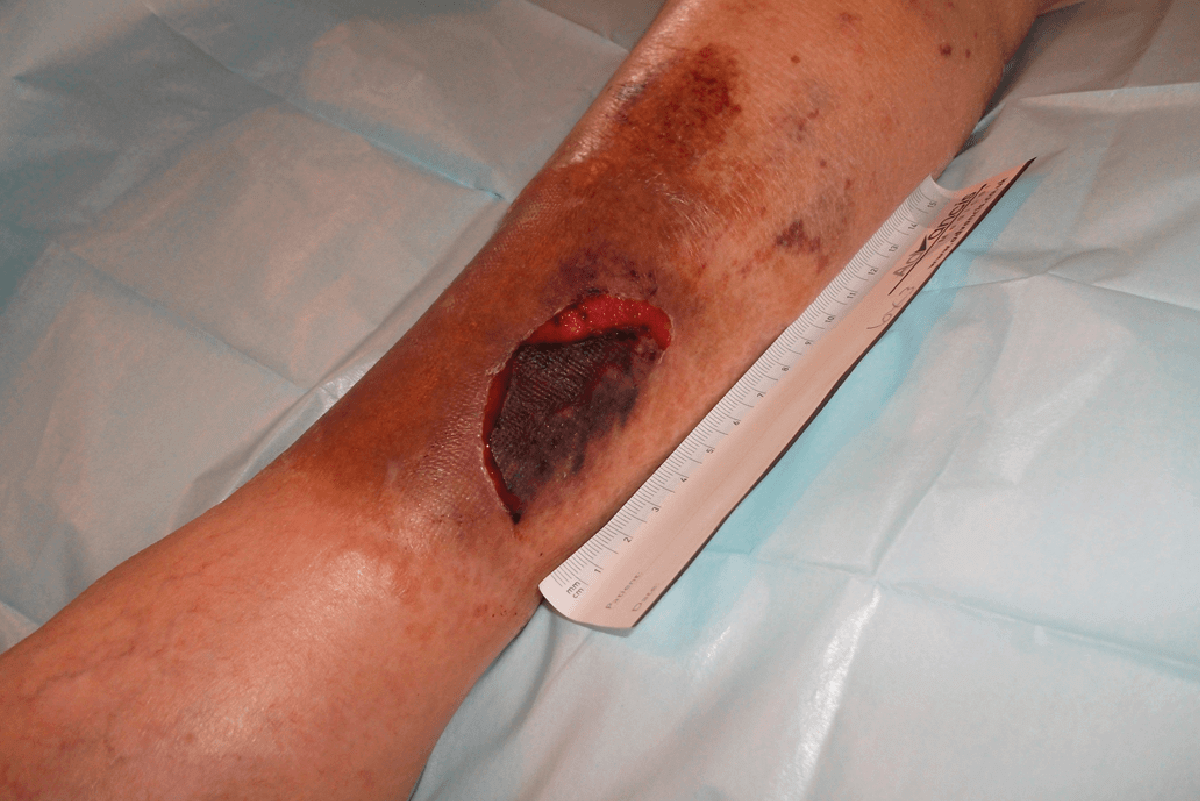
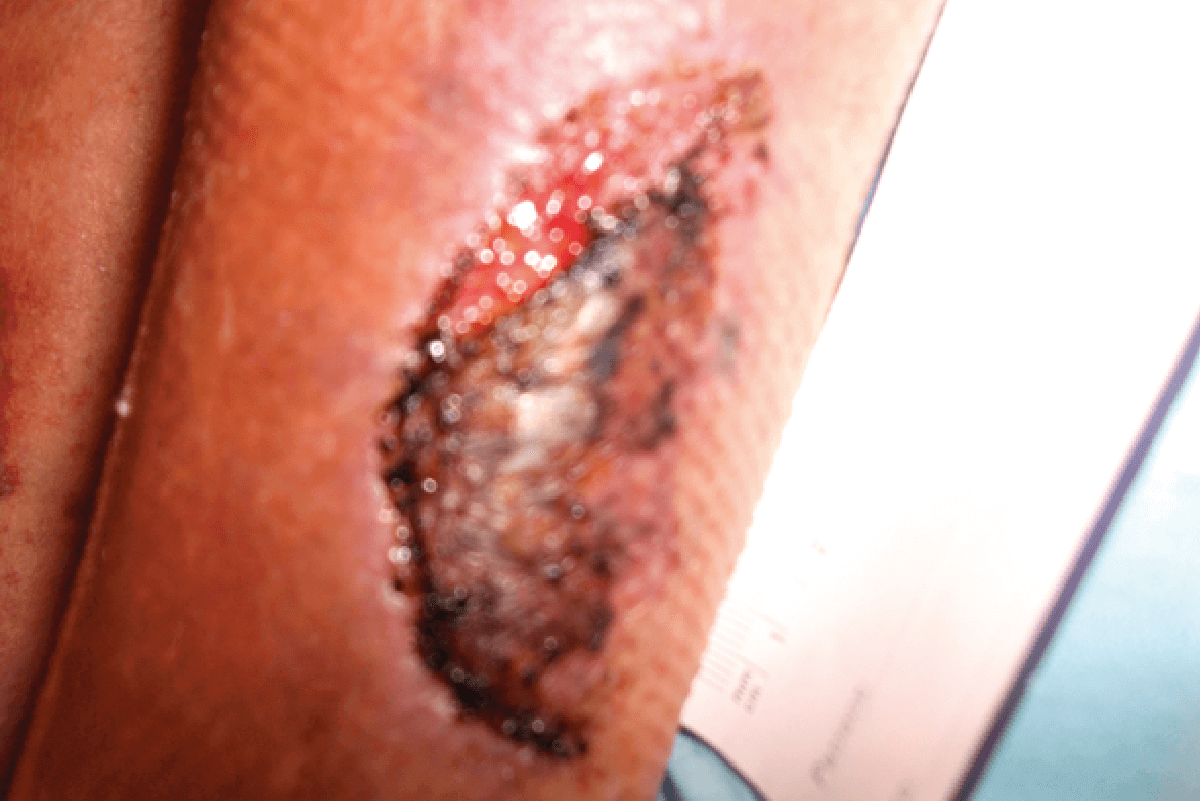
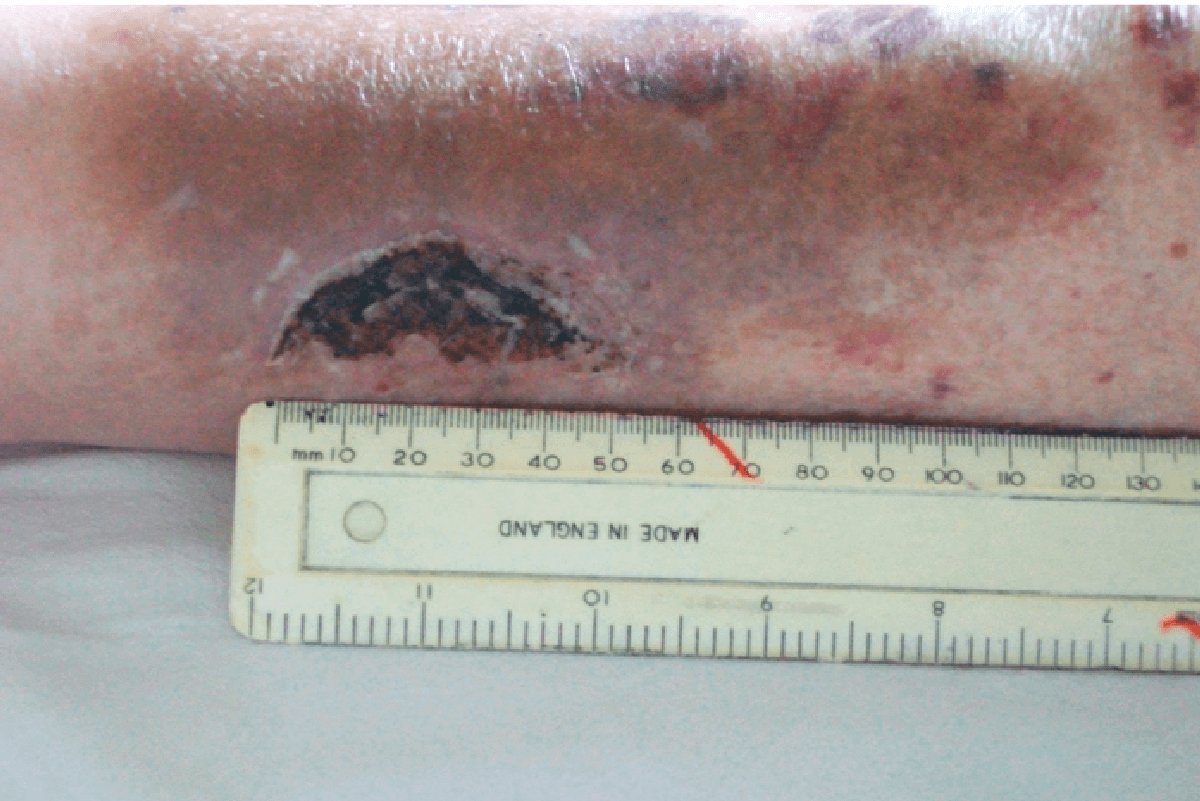
FREE TWO WEEK EVALUATION
ACTIVELY TRY FOAM ADHESIVE OR FOAM NON-ADHESIVE.
*Health care professionals only
* Required Field

SIZES & CODES
| ActivHeal® | FOAM NON-ADHESIVE | |
|---|---|---|
| Size (CM) | QTY | Product Code |
| 5x5 | 10 | 10009113 |
| 7.5x7.5 | 10 | 10009114 |
| 10x10 | 10 | 10009115 |
| 10x20 | 10 | 10011265 |
| 20x20 | 10 | 10009117 |
| 18x12 Heel | 5 | 10010227 |
| 10x10 Tracheostomy | 10 | 10009118 |
| FOAM HEEL | ||
| 18x12 | 5 | 10010227 |
| FOAM TRACHEOSTOMY | ||
| 10x10 | 10 | 10009118 |
1. SMTL Independent Data Report No. 10/3421/1.
Tegaderm is a registered trademark of 3M.
Allevyn is a registered trademark of Smith & Nephew.
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd